
Primary efficacy outcomes were (a) MACE, defined as CV death, myocardial infarction or ischemic stroke and (b) a composite of cardiovascular death or hospitalization for heart failure. Eligible subjects had an HbA1c level between 6.5 and 12.0% as well as a creatinine clearance of 60 ml or more per minute. Subjects with type 2 diabetes, aged 40 years or older, who had or were at risk for atherosclerotic CV disease, were randomly assigned to receive, in addition to their usual antihyperglycemic therapy (which was at the discretion of their treating physician), either dapagliflozin (10mg/day) or a matching placebo.
#DECLARE TRIAL DAPAGLIFLOZIN TRIAL#
The aim of this paper is to propose a state of the art concerning dapagliflozin in the context of the recently published DECLARE-TIMI 58 Trial (for Dapagliflozin in cardiovascular Events-Thrombolysis In Myocardial Infarction 58) (14). Observational data, especially in the CVD- Real study, are in phase with the results of these two important randomized trials published in the New England Journal of Medicine (10-13).ĭapagliflozin (Forxiga®) is a selective inhibitor of glucose cotransporter 2 that blocks glucose resorption in the proximal tubule of the kidney, promoting thereby glycosuria, weight loss and reduction in blood pressure levels (9). Moreover, as demonstrated in the EMPA-REG OUTCOME (10) and CANVAS (11) studies, in type 2 diabetic patients who had (in majority) established CV disease, they are associated with a significant reduction in CV events, in particular in the hospitalization rate for heart failure (approximatively -40% vs. SGLT-2 inhibitors are glucoretic agents characterized by a high antihyperglycemic efficacy in the setting of normal renal function (8,9). In this context, antihyperglycemic agents with a collateral “cardio protective” effect in terms of major adverse cardiovascular (CV) events (MACE) and/or heart failure are therefore potentially of main clinical interest in the treatment of type 2 diabetes, as also recently mentioned in the ADA-EASD recommendations (6,7). Conversely, patients with chronic heart failure who developed type 2 diabetes had also a poorer prognosis in terms of overall mortality, as reported by MacDonald et al. only 4% of those diagnosed with diabetes alone (4). Several reports confirmed that survival was lower in diabetic patients in the presence of heart failure: 40% of individuals diagnosed with both diabetes and heart insufficiency will die within the three years vs. As reported recently by Fitchett et al., heart failure has now emerged as a severe complication in diabetic subjects due to atherosclerotic and/or microvascular myocardial changes (4). Type 2 diabetes is associated with an increased risk of vascular complications, in particular macroangiopathy, which is the leading cause of mortality following coronary ischemic disease, stroke or other pathological conditions, including heart failure (1-3). placebo therapy.ĭapagliflozin, DECLARE-TIMI 58, primary and secondary cardiovascular prevention, cardiovascular events, heart failure, nephropathy Article complet :


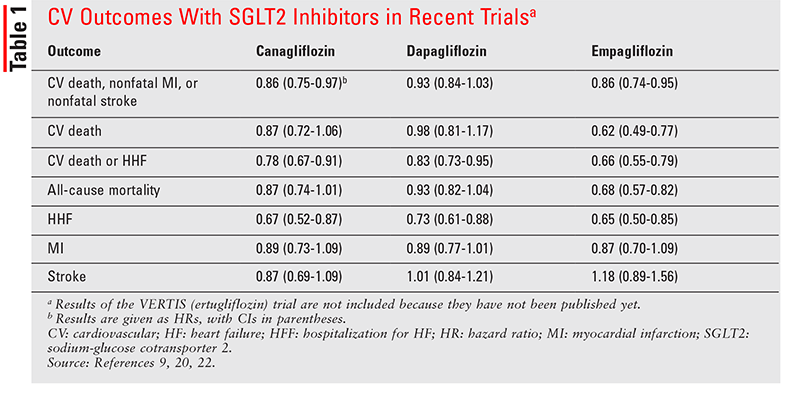
Therefore, this study adds significant clinical information, in addition to the two previous randomized trials with SGLT-2 inhibitors, essentially demonstrating a lower risk for heart failure-induced hospitalizations in patients undergoing dapagliflozin vs. The DECLARE-TIMI 58 was a trial including a broader patient population with about 60% of them in primary cardiovascular prevention. The EMPA-REG OUTCOME and CANVAS trials have both evidenced cardiovascular benefits in Type 2 diabetic patients, with established CV disease in the majority of them. Moreover, the study has revealed a lower risk for renal disease progression. This study involving Type 2 diabetic patients, either with or without prior macroangiopathy, has demonstrated cardiovascular benefits in terms of the primary efficacy outcome, namely a composite of cardiovascular death and hospitalization for heart failure. This paper aimed to review the clinical data on dapagliflozin (Forxiga®) treatment in the light of the recent DECLARE-TIMI 58 trial results.


 0 kommentar(er)
0 kommentar(er)
